Crystal structure: the manner in which atoms, ions, or molecules are spatially arranged.
FCC (face centered cubic)
Atoms are arranged at the corners and center of each cube face of the cell.
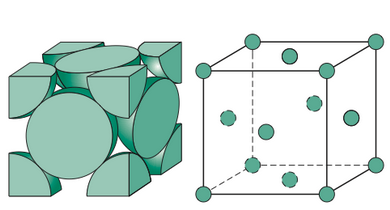
Metals with FCC have high impact energy and they are ductile.
HCP (hexagonal close packed)
Cell of an HCP lattice is visualized as a top and bottom plane of 7 atoms, forming a regular hexagon around a central atom. In between these planes is a half-hexagon of 3 atoms.
There are two lattice parameters in HCP, a and c, representing the basal and height parameters respectively.
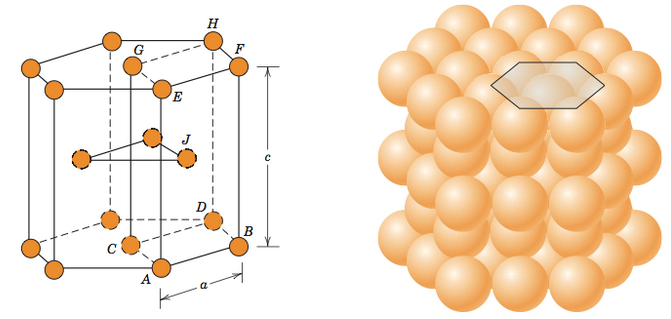
Metals with FCC have low impact energy and they are brittle.
BCC (body-center cubic)
Atoms are arranged at the corners of the cube with another atom at the cube center.
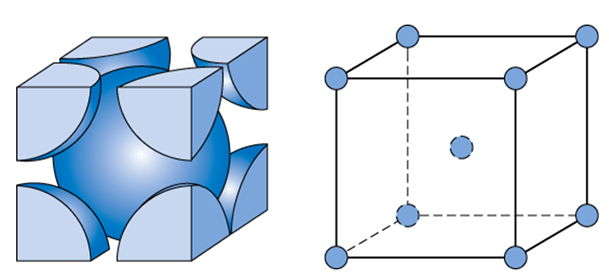
Due to slow dislocation mechanic could be ductile (at high temperatures) and brittle (at low temperatures)
Created: 29 Aug 2022
Last Update: